For difficult-to-treat cancers, like androgen receptor-positive prostate cancer that does not respond to anti-androgen therapy, combination therapy is required. A new study from Drs. Sadar and Tien at the Genome Sciences Centre examined the in vivo response to treatment with both a cyclin-dependent kinase inhibitor and a drug that binds to the N-terminal domain of androgen receptors. Together, palbociclib and an analogue of ralaniten slowed cell division by targeting different checkpoints in the cell cycle.
One in eight men living in North America will develop prostate cancer over their lifetime. In Canada, this equates to an average of sixty-six men diagnosed every day.
Most men diagnosed with prostate cancer respond to therapies aimed at reducing the amount of androgen in the body, primarily testosterone. Approximately 10-20 percent of prostate cancer cases are castration-resistant, however, which means they keep growing even when testosterone is reduced to very low levels.
Targeting the Androgen Receptor
Prostate cancer development and progression is driven by a protein called Androgen Receptor (AR). When bound to dihydrotestosterone or other androgens, AR drives the expression of many genes, including those involved in cell growth.
In some cases of castration-resistant prostate cancer (CRPC), the AR protein is cut in half. This shortened version of AR is capable of turning on genes without binding to androgen, making treatments aimed at reducing androgen levels useless. A truncated version of AR is also detected in some breast cancer cell lines, clinical tissues, and circulating breast cancer cells from breast cancer patients.
To overcome this problem, Dr. Marianne Sadar's lab developed a novel cancer drug that binds to a disordered region of the truncated AR protein. Ralaniten (or the analogue used in this study, EPI-7170) binds to the N-terminal domain–present in both the full length and truncated version of AR—inhibiting AR transcriptional activity and preventing cells from progressing to the S phase of the cell cycle.
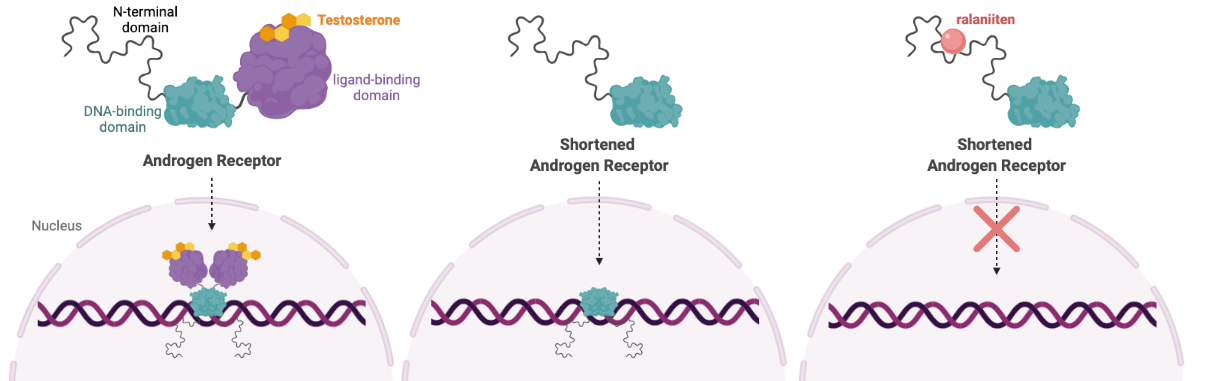
Ralaniten was the first drug that targets disordered proteins to advance to clinical testing, leading to the development of an entirely new class of drugs. Read more about the story behind ralaniten here. Currently, Dr. Sadar’s work has translated into three separate clinical trials in Canada and the USA (NCT02606123, NCT04421222, NCT05075577).
Targeting Cyclin Dependent Kinases
Cyclin-dependent kinases (CDK) are a group of proteins that control the cell cycle. Blocking CDK 4/6 has low toxicity in non-cancer cells because they are not reliant on these kinases for survival. However, cancer cells that express AR are particularly sensitive to inhibitors of CDK4/6.
Clinical studies aimed at treating cancer with CDK4/6 inhibitors only result in short durations of efficacy. To extend remission and potentially lengthen survival times, Drs. Sadar and Tien proposed a double hit on the cell cycle using a specific CDK4/6 inhibitor, palbociclib, in conjunction with ralaniten.
Combining ralaniten and palbociclib therapy
“Monotherapies for metastatic cancers rarely lead to durable responses or cures primarily due to tumor plasticity and heterogeneity with the survival and outgrowth of resistant clones,” says Dr. Sadar. “In this study, we used therapeutics that provided multiple hits on the cell cycle by using a combination of targeting the N-terminal domain of androgen receptor together with palbociclib that inhibits cyclin-dependent kinase 4/6 (CDK4/6). When used together, this combination was predicted to provide a deeper and more durable blockade of proliferation of cancers that express androgen receptor.”
To test this theory, cell-cycle tracing experiments were done on cultured cancer cells that are resistant to antiandrogens (e.g., enzalutamide). Treatment with both EPI-7170 and palbociclib prevented cells in G1 and G2/M from progressing in the cell cycle and caused a portion of cells in the S phase to arrest.
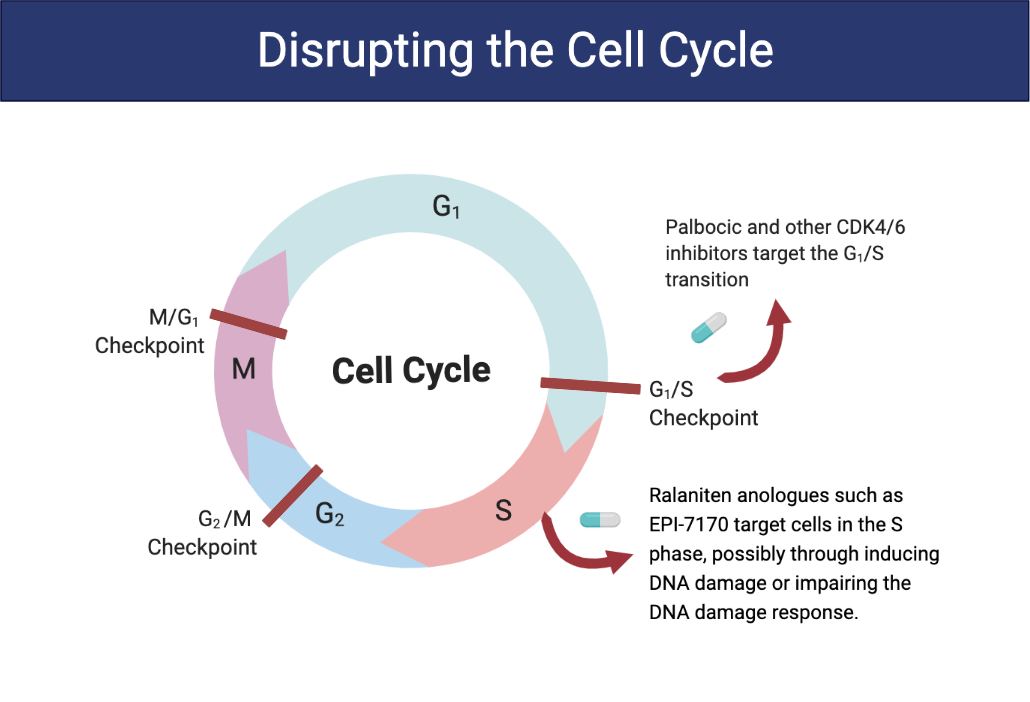
Together, the two drugs contributed to a two-fold increase in doubling time. This means that instead of taking 25 hours for the number of cells to double, it took over 63 hours.
Consistent with this delayed proliferation, in vivo studies in castrated hosts carrying enzalutamide-resistant CRPC xenografts revealed significantly improved antitumor activity with the combination treatment compared to the individual monotherapies.
Better options for hard-to-treat cancer based on molecular characteristics
This is not the first combination therapy Dr. Sadar and her team have studied. Also this year they published that a combination of ralaniten with a PIN1 inhibitor improved in vivo responses for the treatment of castration-resistant prostate cancer by a mechanism that involved multiple hits to the N-terminal domain of AR. Additionally, last year, the group published several papers: 1) showing that their ralaniten analogues synergize with radiation therapy in treatment-resistant prostate cancer and 2) when ralaniten analogues are used in combination with enzalutamide there is a significant antitumour response in some prostate cancers that are resistant to antiandrogens. The latter has been translated into a clinical trial that has just started at two locations in the USA (NCT05075577).
“Our lab has discovered all of the drugs that have been advanced into clinical trials that target the N-terminal domain of androgen receptor,” says Dr. Sadar. “Our goal now is to achieve a more complete and durable response and advance these preclinical studies to the clinic to provide therapy for men with androgen receptor-positive prostate cancer and to those women and some men with androgen receptor-positive breast cancer.”
The next question Dr. Sadar and her team want to explore is what cancers would benefit most from the drug combination based on molecular characteristics. “Hopefully this approach would hold the cancers in check and extend survival times.”
Acknowledgements
This research was supported by funding from the USA National Cancer Institute of the National Institutes of Health.
Banner image credit to National Human Genome Research Institute.
AR and cell cycle images created with BioRender.com
Learn more
Learn more about Dr. Marianne Sadar and the Sadar Lab
Learn more about prostate and breast cancer.
Learn more about research at the GSC.
Citation
Tien, AH & Sadar, MD (2021) Cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with ralaniten analogues for the treatment of androgen receptor-positive prostate and breast cancers. Mol Cancer Ther November 23 (online ahead of print) DOI: 10.1158/1535-7163.MCT-21-0411
*bold font indicates members of the GSC.