Autophagy is a highly regulated process in the cell that removes or recycles unnecessary or dysfunctional molecules, breaking them down and reusing them to assemble new cellular structures. In cancer, it has been shown to contribute to both disease suppression and progression depending on a number of factors, including the stage of the disease. In advanced cancer, it enhances tumour cell survival and resistance to chemotherapy, making it a target for the development of new treatments.
In a new study published in Breast Cancer Research and Treatment, led by GSC Distinguished Scientist Dr. Sharon Gorski, researchers revealed that three key autophagy molecules—ATG4B, LC3B and GABARAP—may be useful together to determine prognosis for breast cancer patients.
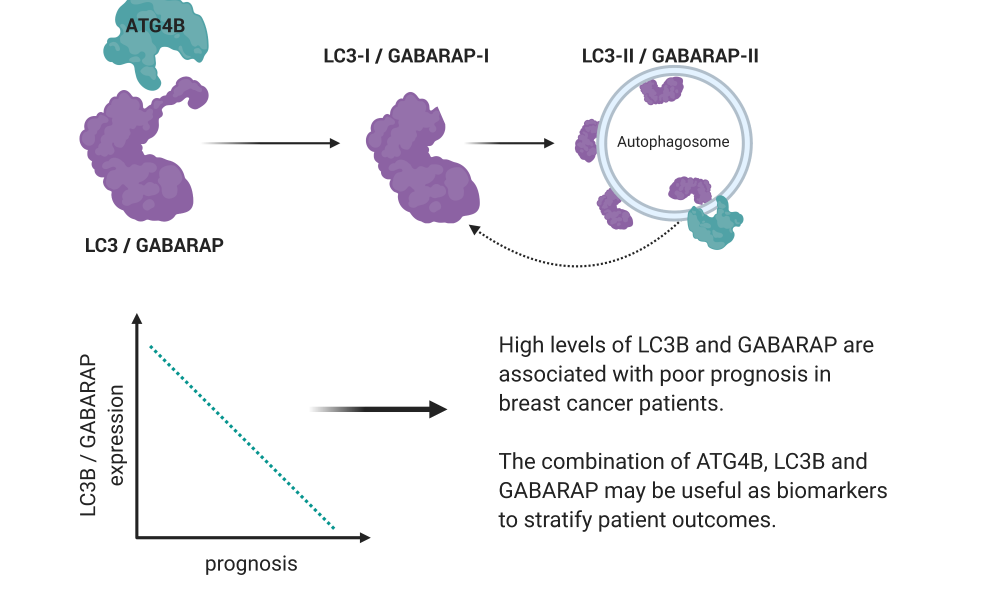
The breakdown and recycling of cellular components is a coordinated effort involving many different proteins. Among these are ATG4B, LC3B and GABARAP which, together with other key players, aid in the formation of membrane-bound compartments called autophagosomes. Cellular components are captured by autophagosomes, which then fuse with lysosomes—another membrane-bound compartment containing enzymes that break down the captured material into their constituent building blocks.
LC3B and GABARAP need to be associated with the autophagosome to carry out their roles in the process. ATG4B is an enzyme that processes LC3B and GABARAP, enabling their attachment to the autophagosome. ATG4B also mediates the removal of LC3B and GABARAP from the autophagosome, allowing them to be reused.
Previous studies have indicated that the dependency on autophagy differs between subtypes of breast cancer and, consistent with this, the GSC research team found subtype-specific differences in expression of these key autophagy proteins. While there were distinct differences in ATG4B expression between breast cancer subtypes, these differences were not significantly associated with patient prognosis. In contrast, high levels of both LC3B and GABARAP were found in basal-like breast cancers and were associated with worse outcomes and markers of aggressive disease among all breast cancer subtypes.
This is the largest study to date analyzing the utility of LC3B, both punctate (membrane-bound) and diffuse staining patterns, as a candidate biomarker for breast cancer prognosis. It was also the first analysis of ATG4B and GABARAP in breast cancer patient tissues. These analyses revealed that the combination of ATG4B, LC3B and GABARAP could potentially serve as a multi-target biomarker to help stratify breast cancer patient outcomes, though further studies are required.
This study was a collaboration between researchers at Canada’s Michael Smith Genome Sciences Centre and Medical Oncology at BC Cancer, the University of British Columbia and Simon Fraser University.
This study was funded by the Canadian Institutes of Health Research, and in partnership with Avon Foundation for Women-Canada.
Learn more about Dr. Gorski’s research.
Learn more about autophagy and cancer research.
Learn more about breast cancer.
Learn more about research at the GSC.