For some prostate cancer patients, radiation and androgen deprivation therapy—a type of chemotherapy that reduces testosterone levels—synergize to enhance treatment outcomes. But in up to 50 per cent of cases, treatment resistance develops, and the cancer progresses.
In a new study published in the journal Cancers, led by GSC Distinguished Scientist Dr. Marianne Sadar, researchers have demonstrated a more effective synergistic treatment strategy, enhancing the efficacy of the combined treatment by targeting Androgen Receptor (AR)—a protein commonly truncated in resistant cases.
 AR is a transcription factor that is activated by testosterone and, once activated, AR proteins pair up and move to the nucleus where they turn on and off a host of genes, affecting cellular function and replication. AR plays a pivotal role in prostate cancer and as a result, many treatment strategies target this protein.
AR is a transcription factor that is activated by testosterone and, once activated, AR proteins pair up and move to the nucleus where they turn on and off a host of genes, affecting cellular function and replication. AR plays a pivotal role in prostate cancer and as a result, many treatment strategies target this protein.
One way to reduce the effects of AR gone rogue is to prevent its interaction with testosterone. This can be done using drugs or procedures that reduce levels of testosterone or by physically preventing it from binding to AR.
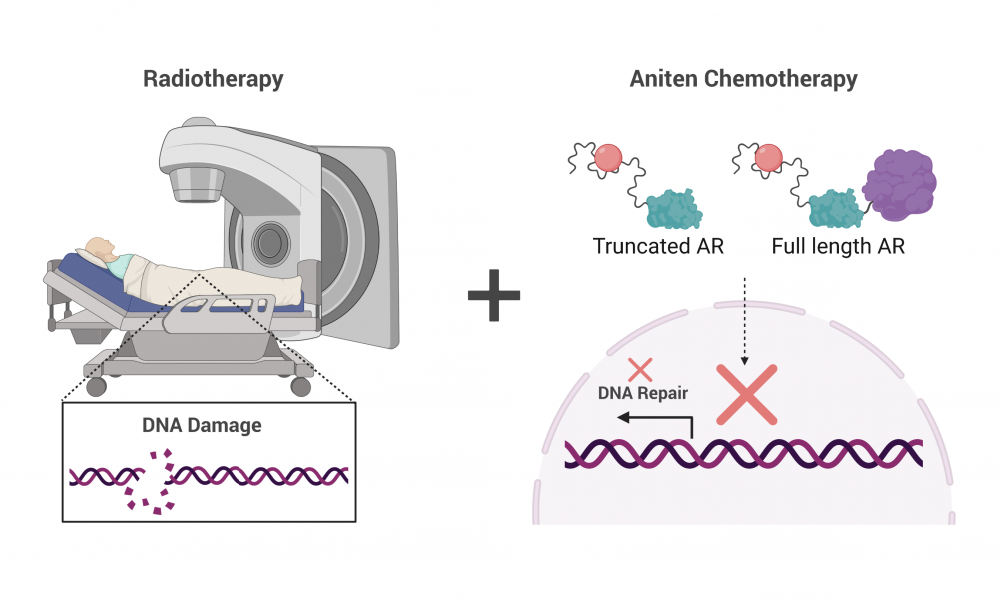
Studies have shown that drugs targeting AR synergize with radiation therapy to enhance treatment outcomes. Radiation therapy works by damaging DNA, leading to cancer cell death. But AR regulates the expression of genes involved in repairing damaged DNA. Thus, blocking the activity of AR prevents DNA repair, enhancing the DNA damaging effects of radiation therapy.

Unfortunately, some men develop resistance to this treatment strategy and in some of these cases, AR is spliced so that the AR protein is cut in half. And it turns out, the shortened version functions independent of testosterone, rendering testosterone-blocking drugs useless. Upon treatment with radiation therapy, the shortened AR turns on expression of DNA repair genes, thereby reducing the efficacy of radiation.
“In advanced prostate cancer, the AR is truncated,” says Dr. Sadar. “So now you have an AR that is always active, always turning on genes. All of the therapies being given to these patients are targeting a part of the protein that isn’t even there.”
To circumvent the activity of shortened AR, Dr. Sadar developed a class of drug—called anitens—that are the only drugs known to block both the full length and the shortened protein, making them a promising treatment strategy for resistant cases.

In this study, Dr. Sadar’s team investigated the synergistic effects of combining their drug, ralaniten, and its improved derivatives with radiation therapy. To do this, they treated human prostate cancer cells expressing both full-length and shortened AR with the drugs. They found that drug treatment reduced expression of DNA repair genes and that it had an additive effect when combined with radiation.
These findings build upon an extensive body of research conducted in the Sadar lab and provide further evidence that targeting the shortened form of AR is a promising avenue for men with treatment-resistant prostate cancer.
Carmen A Banuelos, Yusuke Ito, Jon K Obst, Nasrin R Mawji, Jun Wang, Yukiyoshi Hirayama, Jacky K Leung, Teresa Tam, Amy H Tien, Raymond J Andersen, Marianne D Sadar. Ralaniten Sensitizes Enzalutamide-Resistant Prostate Cancer to Ionizing Radiation in Prostate Cancer Cells that Express Androgen Receptor Splice Variants. Cancers.
This work is supported by the National Cancer Institute of the National Institutes of Health.
Learn more about Dr. Marianne Sadar and her Research
Learn more about the Sadar Lab
Learn more about Research at the GSC
Learn more about Prostate Cancer
Figures created using biorender.com